Key hypothesis and expected outcomes
The overall aim of this proposal is to gain insight in the natural history of GATA2 deficiency through building a unified data set collection at European level and developing preclinical scientific models to delineate personalized follow-up and treatment for patients.
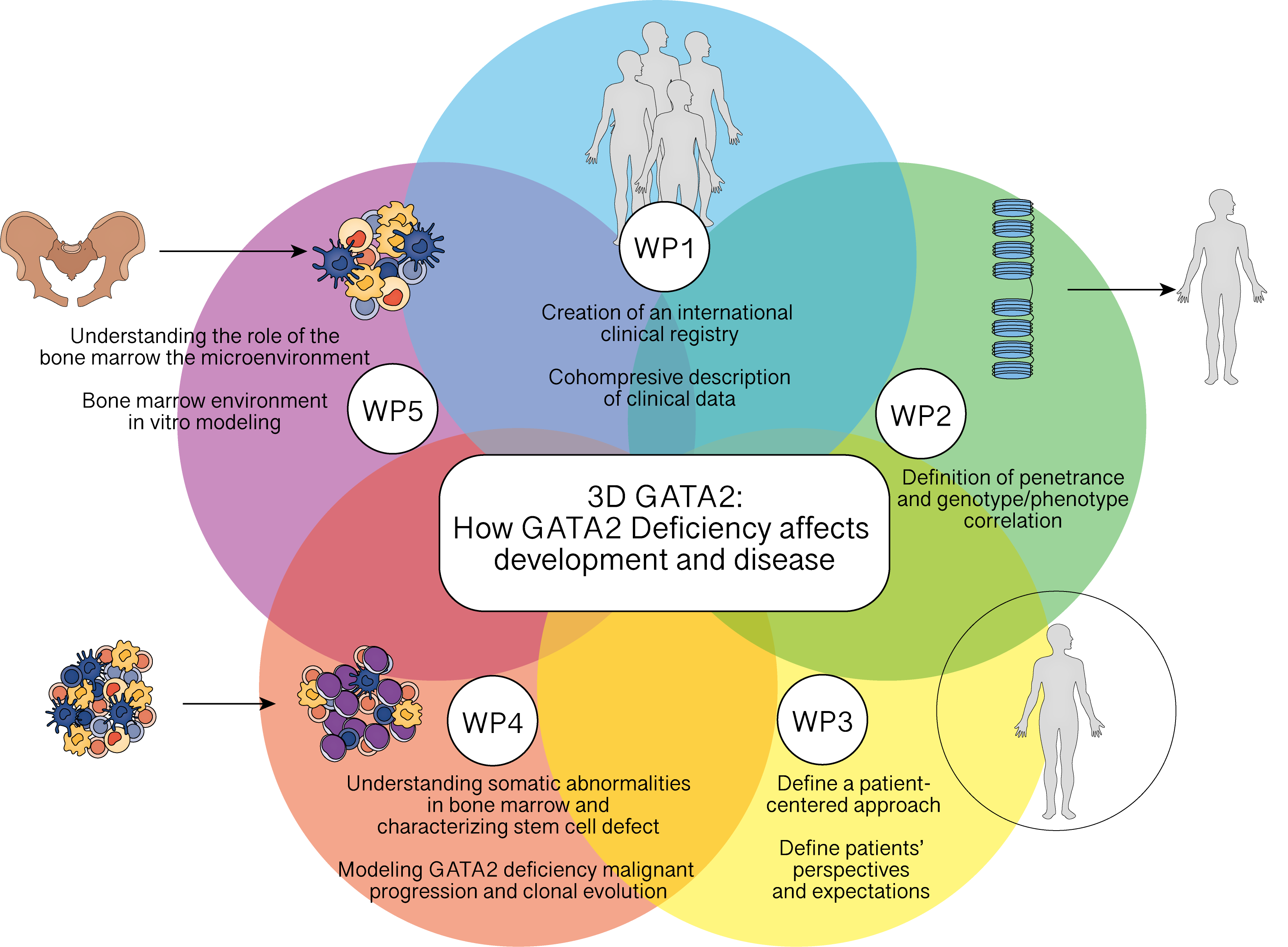
Ongoing Projects and Research Areas
This consortium’s research is split in 6 main work packages with distinct objectives:
Work Package 1
Unified data set and biosamples to gain insight in natural history of GATA2 deficiency
To draw a precise picture of clinical manifestations and natural history of GATA2 deficiency building on different large national and international patient registries
Work Package 2
Genotype/phenotype correlation of major clinical manifestations in GATA2 deficiency
To determine genotype-phenotype correlation in GATA2 deficiency and the penetrance of major clinical manifestations
Work Package 3
Patients’ perspectives and expectations regarding follow-up and Quality of Life
To address the GATA2 deficient patients’ perspectives and expectations regarding patient follow-up and Quality of Life (QoL)
Work Package 4
Evaluation of disease risk and progression to leukemic stages by single-cell multi-omic approaches
To delineate the mechanisms of malignant transformation in GATA2 deficiency to identify prognostic markers, therapeutic targets and preventive measures
Work Package 5
Establish in vitro and in vivo GATA2-deficient models to assess the functional relevance of distinct alterations and drug effectiveness
To establish preclinical GATA2 disease models to study mechanisms of malignant transformation, the contribution of niche cells and new therapeutic options
Work Package 6
Implement personalized medicine into the care of patients with GATA2 deficiency
To establish algorithms for patient-tailored diagnostic and therapeutic management
3D GATA2 research overview
Work Package 1
Unified data set and biosamples to gain insight into the natural history of GATA2 deficiency
Members of the consortium possess substantial expertise in establishing registries in their respective countries. Pasquet, coordinator of the consortium, leads the GATA2 registry in France, which has registered up to 150 French and Belgian patients recruited in 2023. Together with our German partner (Erlacher/Platzbecker, 40 patients), Spanish partner (Giorgetti, Catala, Bigas, 20 patients), and Italian partner (Masetti, 40 patients). Inclusion per year was estimated to 30 patients/year in the consortium. In total, we plan to include around 300 to 350 GATA2-deficient patients, with the majority having complete phenotype descriptions, and some of them having national biological sampling. Genotype/phenotype correlation will be estimated based on the available dataset.
Inclusion criteria
1. All subjects with a pathogenic or likely pathogenic germline GATA2 mutation or deletion.
2. Signed written informed consent at the national level.
Exclusion criteria
1. GATA2 somatic mutation.
2. Any psychological, familial, geographic or social situation, according to the judgment of the investigator, potentially preventing the provision of informed consent
3. Person who has forfeited his/her freedom by administrative or legal award or who is under legal protection, with the exception of persons under curatorship
Anonymisation/pseudonymisation of data
The main objective is to pool data from multiple sources to provide a unified dataset for other WPs. Clinical data of interest will be defined collectively by the project leader of each WP and the Biostatistics & Health Data Science Unit (B&HDS). Each partner will be responsible for pseudonymizing the individual patient data. The Pseudo ID will serve as the unique patient reference for any dataset transmitted to the B&HDS within the project.
Work Package 2
Defining the Clinical Spectrum and Hematological Risk in GATA2-Deficient Patients
GATA2 deficiency is a complex genetic disorder with highly variable clinical manifestations. To date, no strong correlation has been established between specific GATA2 variants and clinical phenotypes, age of onset, or long-term outcomes. This variability underscores the need for a comprehensive assessment of both symptomatic and asymptomatic carriers to define genotype-phenotype correlations and estimate age-specific risks for disease development.
Building on the foundation of the international patient registry established in WP1, WP2 focuses on systematically characterizing the clinical spectrum of GATA2 deficiency. By leveraging data from a large, diverse cohort, we aim to clarify the penetrance of specific symptoms and assess potential gender-specific differences in disease presentation.
Our Approach
To achieve these objectives, we will analyze data from patients with confirmed GATA2 mutations, categorizing them based on hematological, immunological, and other clinical features. The primary focus will be on:
-
Hematological Features: Subdivided into bone marrow failure (BMF)/cytopenia, myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML), and patients without hematological abnormalities.
-
Immunological Features: Including patients with infections characteristic of GATA2 deficiency, those with an immunological phenotype (NK-cell reduction, B-cell deficiency, dendritic cell/monocyte depletion), and those without overt immune dysfunction.
-
Other Clinical Manifestations: Encompassing non-hematological and non-immunological features such as deafness, lymphedema, and pulmonary alveolar proteinosis.
Statistical Analysis and Impact
By employing Kaplan-Meier survival analysis and age-specific cumulative risk estimation, we will assess the likelihood of developing clinical manifestations over time. The results of this study will significantly enhance our understanding of the natural history of GATA2 deficiency. By defining the clinical trajectory of affected individuals, we aim to refine diagnostic criteria, improve risk assessment strategies, and ultimately inform personalized clinical management approaches for patients with GATA2 mutations.
Work Package 3
Patients’ Perspectives & Quality of Life
Understanding the patient experience is essential in improving care for GATA2 deficiency. Work Package 3 focuses on gathering insights from patients and caregivers regarding their medical journey, expectations for follow-up care, and overall quality of life. By incorporating their perspectives, we aim to improve awareness, enhance follow-up care, and ensure that patient experiences shape future research and treatment strategies.
A key aspect of this work is a tailored survey designed specifically for patients with GATA2 deficiency and their caregivers. Developed with input from our Steering Committee and patient focus groups, the survey will provide valuable insights into the challenges they face. The findings will be analysed and published in a scientific article to contribute to the broader understanding of patient needs and outcomes by the scientific and medical communities.
Additionally, a Patient Journey Guideline will be developed to outline the key stages of diagnosis, treatment, and follow-up care. This resource will help both patients and healthcare professionals navigate the condition more effectively.
To ensure the findings reach the right audiences, we are committed to widespread dissemination. A dedicated project website will provide regular updates on progress, resources, and results for both medical professionals and patients. We will also organize a webinar in collaboration with expert networks, such as the European Reference Network for Rare Immunological Disorders (ERN-RITA), European Working Group (EWOG), and European Society for Immunodeficiencies (ESID), to engage the medical and research communities. To further raise awareness, we will create a targeted leaflet for physicians unfamiliar with GATA2 deficiency, which will help improve diagnosis and patient management.
By placing patients at the heart of research, Work Package 3 ensures that their voices are heard, their challenges addressed, and their quality of life improved.
Work Package 4
Defining the Clinical Spectrum and Hematological Risk in GATA2-Deficient Patients
Understanding somatic abnormalities in patients’ bone marrow. We have shown that some abnormalities were enriched in patients with an excess of blasts (e.g., SETBP1 mutations) while others (e.g., STAG2 mutations) were more in favour of a clonal haematopoiesis with a low transformation risk. Similarly, monosomy 7 is associated with a high risk of disease progression, and most patients with MDS and excess blasts harbor monosomy 7.
We will extend these NGS analyses to the whole patient cohort of this consortium and correlate the somatic landscape with clinical outcomes in a longitudinal manner. In addition, we will employ a single-cell integrative genotyping and chromatin accessibility approach to identify cells with somatic abnormalities and understand the consequences compared to cells mutated only for GATA2.
These studies will be used to identify molecular patterns indicating imminent leukemia risk that can be used for therapeutic decisions.
Characterizing stem cell defect
Our preliminary results in a KI Gata2R396Q/+ mouse model showed heterogeneity in the expression of the mutated Gata2 allele in the LT-HSC stem compartment (Largeaud et al., 2024). A high level of expression of the mutated allele results in a strong hypo-functionality of these cells. In patients, we found a decrease in some stem populations (GMP and CMP) and a persistence of the MEP population. However, to date, no study has demonstrated a correlation between the phenotypic and transcriptomic profiles. Using a single-cell transcriptomic approach, we aim to demonstrate a correlation between deregulated transcriptomic signatures and the ratio of mutated allele to wild-type allele expression. By this approach, we will be able to use surface proteins as a prognostic marker in this cohort.
Work Package 5
Establish in vitro and in vivo GATA2-deficient models to assess the functional relevance of distinct alterations and drug effectiveness
The goal of this WP is to develop advanced humanized in vitro and in vivo models that accurately replicate the genetic complexity of GATA2 deficiency. These models will enable us to study clonal evolution, investigate the role of the bone marrow (BM) microenvironment, and assess potential therapeutic agents in a GATA2-specific context.
While most research on GATA2 deficiency has focused on blood components, the BM niche—the supportive environment surrounding blood cells—has been largely overlooked. However, previous studies suggest that an abnormal niche may act as a “fertile soil” for malignant cell expansion in myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), potentially driving disease progression. Understanding these interactions is crucial, as specific alterations in the BM niche may predispose individuals to disease initiation and evolution.
Our Approach
Using GATA2-deficient cells, 3D co-culture systems, OMICs analysis, and animal models, we aim to uncover how hematopoietic stem/progenitor cells (HSPCs) interact with the non-hematopoietic components of the BM niche. Our key research steps include:
-
Isolating mesenchymal stem cells (MSCs) and endothelial cells (ECs) from BM biopsies of GATA2-deficient patients.
-
Use these cells for 3D-coculture
-
Characterizing the cross-talk between hematopoietic and non-hematopoietic cells through in vitro functional studies and multi-omics analyses (transcriptomics, proteomics, and metabolomics).
-
Use our vitro/vivo models to test different combinations of drugs such as AZA, venetoclax, navitoclax and magrolimab
Impact
By investigating how GATA2 mutations reshape the BM microenvironment and contribute to malignant transformation, we can pave the way for:
- More effective treatment strategies
- Improved transplant outcomes
- A deeper understanding of disease progression
Project Milestones
1. National Patient Registries
- Clinical data of interest
- Unified Data Set
- Pseudo-anonimization
- Data collection and curation
Until May 2026
2. Genotype
/phenotype correlation in GATA2 deficiency
- Analysis of clinical variables collected
- Analysis of the correlation between genotype and phenotype of the clinical
variables - Assessment of the penetrance of specific symptoms associated with
particular GATA2 variants
Until Dec 2026
3. Evaluation of disease risk and progression to leukemic stages by single-cell multi-omic approaches
- Single-cell DNA sequencing
- Bioinformatical analyses
Until Dec 2026
4. Establish in vitro and in vivo GATA2-deficient models
- Characterization of BM microenviroment in KI GATA2 mice and GATA2 patient
- GATA2+/- mouse model to study mechanisms of leukemogenesis
- PDX model (with GATA2 patient cells)
- Drug testing
Until Dec 2026
5. Plan to implement personalised medicine for GATA2-deficiente patients
Until Aug 2026
6. Patients
- Website
- Panel
- Survey GATA2 Deficient Patients and Caregivers
- Patient Journey Guide
- GATA2 deficient patients’ self-reported outcomes Paper
- Leaflet featuring GATA2 and project
- Webinar featuring project outcomes

